
Anti-Dupilumab antibody
Anti-Dupilumab is a chimeric rabbit/mouse anti-idiotypic antibody targeting the therapeutic antibody Dupilumab. It has rabbit variable domains and mouse constant domains and mainly binds to free Dupilumab in samples.
| Article number | M9263 |
| Product group | Antibody |
| Technique | ELISA |
General information
Description:
Anti-Dupilumab is a chimeric rabbit/mouse anti-idiotypic antibody that specifically targets the human therapeutic antibody Dupilumab. It consists of rabbit variable domains and mouse constant domains. This antibody mainly binds to free Dupilumab in samples.
Target:
Dupilumab is a human IgG4/kappa antibody directed at both the interleukin-4 (IL-4) and IL-13 receptors, blocking their signaling pathways.
Application
Anti-Dupilumab antibody has been evaluated in ELISA. Validation in other techniques is required by the user. It is recommended to test the antibody by titrating the product in the chosen technique, using appropriate negative and positive controls.
For research use only!
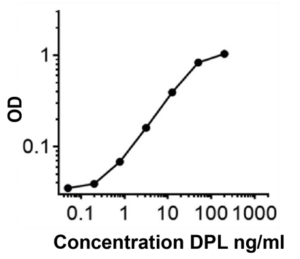
Figure
Figure 1: Titration of Dupilumab to create a pharmacokinetic (PK) curve in bridging ELISA.
Anti-Dupilumab antibody was used as capture (0.5 µg/ml) and detection antibody (0.250 µg/ml) in
sandwich assay format.(5) DPL= Dupilumab
AntiBodyChain is the official worldwide distributor for the Sanquin/Essange Reagents products.
References
Find out more information about the scientific background of the product.
- European Medicines Agency (EMA). Dupixent (dupilumab).
https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent. 2024. - Bosma AL, Gerbens LAA, El Khattabi H, Loeff FC, Duckworth M, Woolf RT et al. The clinical relevance
of dupilumab serum concentration in patients with atopic dermatitis: a two-center prospective
cohort study. Journal of Dermatological Treatment 2023; 34. PMID:37098906. - Spekhorst LS, De Graaf M, Loeff F, Zuithoff NPA, Bakker D, Boesjes CM et al. Association of Serum
Dupilumab Levels at 16 Weeks with Treatment Response and Adverse Effects in Patients with
Atopic Dermatitis: A Prospective Clinical Cohort Study from the BioDay Registry. In: JAMA
Dermatology. American Medical Association, 2022, pp 1409–1413 PMID:36322072. - Spekhorst LS, Bakker D, Drylewicz J, Rispens T, Loeff F, Boesjes CM et al. Patient-centered dupilumab
dosing regimen leads to successful dose reduction in persistently controlled atopic dermatitis.
Allergy: European Journal of Allergy and Clinical Immunology 2022; 77: 3398–3407. PMID:35837880. - Großerichter-Wagener C, Kos D, van Leeuwen A, Dijk L, Jeremiasse J, Loeff FC et al. Biased anti
idiotype response in rabbits leads to high-affinity monoclonal antibodies to biologics. MAbs 2020;
12. PMID:32887534.
Partners AntiBodyChain


Are you interested in a product or do you have questions?
Fill in the information below.
